CSEC Physics Syllabus - Effective for examinations from May - June 2015
Section A - Mechanics
Scientific Method
Specific Objective 1.10
apply the formula for density: ρ = m/V ;
 |
| Pixabay |
Density
Definition :
- Density is the mass per unit volume of an object.
- It is the mass in a given volume of an object.
Symbol : ρ (rho)
S.I. Unit : kilogram per metre cube - kg/m3
Other Unit : gram per centimetre cube - g/cm3
Other Unit : gram per centimetre cube - g/cm3
Table 1 showing the densities
of some useful substances.
Substance
|
Density
in gcm-3
|
Density
in kgm-3
|
Helium
|
0.00017
|
0.17
|
Air
|
0.0012
|
1.2
|
Gasoline
|
0.8
|
800
|
Pure
Water
|
1.0
|
1
000
|
Aluminum
|
2.7
|
2
700
|
Steel
|
7.92
|
7
920
|
Copper
|
8.9
|
8
900
|
Glass
|
2.5
|
2
500
|
Soft
Wood
|
0.5-0.6
|
500-600
|
Gold
|
19.3
|
19
300
|
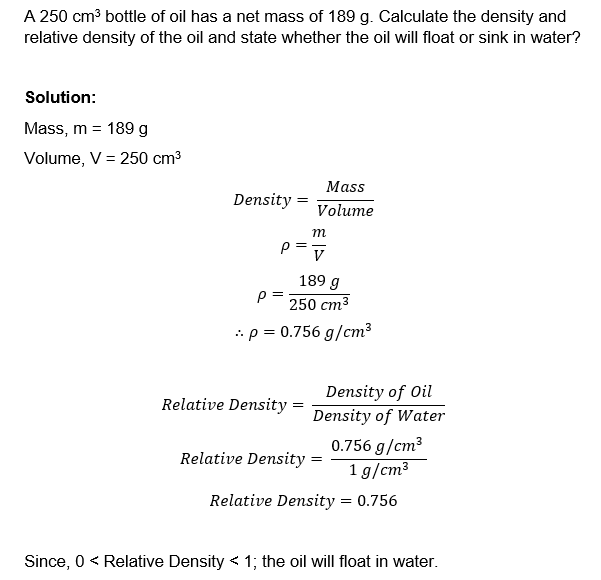
Example 1
Relative Density is a ratio of two densities; in most cases the density of a material or substance compared to the density of water.
Relative Density measures how many times a substance is more dense than water. It is a dimensionless quantity, which means it has no units.
Formula : Relative Density = Density of Substance ÷ Density of Water
Note:
If Relative Density ≥ 1 : the substance/material will sink
If 0 < Relative Density < 1 : the substance/material will float
Relative Density measures how many times a substance is more dense than water. It is a dimensionless quantity, which means it has no units.
Formula : Relative Density = Density of Substance ÷ Density of Water
Note:
If Relative Density ≥ 1 : the substance/material will sink
If 0 < Relative Density < 1 : the substance/material will float









2 Comments
Cool tutorial
ReplyDeleteThanks! Don't forget to share 👍🏽
Delete